Download PDF format
PREVALENCE OF GASTROINTESTINAL NEMATODES OF FARM ANIMALS BY COPRO-CULTURE
Mohammad Saleh Al-Aboody1, Mosaab A. Omar2
1College of Science Al-Zulfi, Majmaah University, Saudi Arabia
2Department of Parasitology, College of Veterinary medicine, South Valley University, Sohag, Egypt.
Abstract
In the present study, examination of 442 faecal samples was performed: 171 from cattle, 128 from buffaloes and 143 from sheep. During the period from May, 2014 to April, 2015, fecal examination showed the infection rate with abomasal nematodes was 30% in cattle, 22.6% in buffaloes, and 31.4% in sheep. Fecal culture gave results of 47.5%, 30%, and 50.3% in cattle, buffaloes and sheep respectively. Seasonal infection with abomasal nematodes as shown by faecal culture in cattle, reveals the highest infection rate is in summer (55.9%), followed by spring (54.1%), autumn (50%), and winter (33.3%). Cooperia spp. is the most prevalent larva in both cattle and buffaloes; Strongyloides papillosus is the most predominant one in sheep. Here we introduce the first study of abomasal worms infection in ruminants in Sohag, Egypt. The prevalence is found to be so high among the all examined animals, that we recommend that the authorities apply suitable control programs.
Keywords: Haemonchus, Ostertagia, seasonal dynamics, floatation.
Introduction
RUMINANTS comprise the greatest portion of animal stock in Egypt’s Sohag province. The gradual growth in the human population with its increased consumption of animal protein has put a strain on the limited current animal production. More research into the problem of animal production is needed (Sultan et al. 2010; Perry et al. 1998; Guirgis 1984 and Hanelein and Abdellatif 2003). The parasitic infections that afflict the animal production industry have great economic impact, especially in developing countries (Sultan, et al. 2010 and Perry et al. 1998). Richard and Zimmerman (1992) investigated the epizoology of gastrointestinal nematodes in selected areas of Oregon, USA. They identified eight nematode genera, among them Ostertagia, Cooperia, Trichostongylus, and Capillaria. Khalaf-Allah (1994) reported that the most common nematodes among cattle and buffaloes in Kalubia governorate were Ostertagia, Haemonchus, Trichostrongyle, and Cooperia. He concluded that nematodes infection was generally more severe in young animals, the infection rate decreasing as the animal ages. Professional livestock production is an important business and anything that adversely affects production will impair the economy, hurting the individual farmer as well as the entire industry (Corwin 1997). The aim of the present work is to investigate gastro-intestinal nematodes that infest ruminants in Sohag province, their incidence, prevalence, and seasonal fluctuation.
Materials and methods
Ruminants of different ages and sexes were investigated during a period from May, 2014 to April, 2015. We looked for the presence of helminthes, their incidence, and seasonal activity. A total of 442 faecal samples from cattle, buffaloes, and sheep were examined. Samples were examined from cattle (171), from buffaloes (128), and from sheep (143). The faecal samples were collected in situ from different places and farms, as well as some brought to the clinics from Sohag, Egypt (26° 1012, N 32° 4337 E).
A. Collection of Faecal Samples
Faecal samples were collected directly from the rectum or immediately after defecation in plastic sacs, each labeled with the data as to age, sex, and date of collection, locality, and the presence of any apparent lesions. Then, the samples were transferred to the Parasitology Laboratory of the Faculty of Veterinary Medicine in Sohag. The samples were collected throughout the year.
B. Preparation and Examination of Faecal Samples
Preparation and examination of the faecal samples was done according to Burger and Stoy (1968) and Charles (1998).
C. Gross Examination of Faeces
1. Flotation Technique
The flotation technique with saturated salt solution was used to detect the nematode and cestode eggs Burger and Stoy (1968).
2. Faecal Culture Technique
Fecal culture is used in diagnostic parasitology to differentiate parasites whose eggs can’t be easily distinguished. To distinguish between trichostrongyle eggs, for example, fecal culture must be used.
The technique of faecal culture was carried out using standard methods described by (Burger and Stoy 1968; Eckert 1960 and Abdel Gawad 1972).
D. Data Management and Analysis
All the data that were collected (age, species, and parasitic infestation) was entered on an MS Excel sheet and analyzed using SPSS version 16. Descriptive statistics were used to determine the prevalence of the disease and the χ2-test was used to look for the significant correlations between host age and host species with the parasites prevalence.
Results
The present study using fecal examination revealed that the infection rates with abomasal nematodes in cattle was 30%, in buffaloes 22.6%, and in sheep 31.4%. Using fecal culture, the results were 47.5%, 30%, and 50.3%, respectively (Table I).
The monthly infection rate with Trichostrongyles found in cattle using faecal culture (Table I), was highest in June (68%), March (66.7%), November (53.3%), October (52.9%), and in April (50%). In buffaloes the highest infection rates were recorded in March (58.3%), November (50%), April (46.1%), and October (44.4%). Finally, in sheep the monthly infection rates were the highest in June (66.6%), May (60%), July and January (58.3%), April (56.2%), and October (54.5%).
Variation of Trichostrongyles infection in cattle by season (Table II) revealed that the highest infection rates occurred in summer (55.9%) and in spring (54.1%), while the lowest rate of infection occurred in winter (33.3%). In buffaloes, the highest infection rates occurred in spring (47%), autumn (42.8%), and summer (28%) respectively. The lowest rate of infection was in winter (21.9%). In sheep, the highest helminthes infection rates occurred in spring (55%), followed by summer (53.1%), autumn (52.5%), and then winter (40 %).
Concerning the presence of different G.I. larvae found in cattle, buffaloes and sheep after faecal culture (Table III), the results revealed that the most prevalent larvae in cattle were Cooperia (49.3%), followed by Strongyloides papillosus (40.9%) Ostertagia spp. (26.5%) and Haemonchus (18%) Figs. 1 and 2. Trichostrongylus spp. Figs. 3, 4 and Oesophagostomum spp. were the lowest prevalence, at 15.6% and 6% respectively. In buffaloes, the most prevalent larvae were Cooperia (45%), Strongyloides papillosus (43%), Ostertagia spp. (34%) and Trichostrongylus spp (25%). Haemonchus pp was least prevalent, at 9%. In sheep, the most prevalent larvae were Strongyloides papillosus (37.5%), Ostertagia spp. (30.5%), Oesophagostomum spp (27.7%) and Haemonchus spp. (26%). Least prevalent was Trichostrongylus sp. (12%).
Discussion
The present study revealed that the infection rate was highest in sheep while buffaloes showed the lowest level of infection. This may be due to the fact that buffaloes have been shown to be more resistant to parasitic infestations than other ruminants Ezzat (1996). That the infestation in sheep was found to be the highest among all the animal examined agrees with the observations of (Fakae 1990; Torina et al 2004; and Chollet et al 1994).
Concerning the seasonal infection rates with trichostrongyles larvae in cattle, buffaloes, and sheep in Sohag province, the summer season is the most favorable one for cattle, with a peak in June, then spring, with a peak in March, and autumn, with a peak in November. But the variance among the three seasons is small, due to the similarity of environmental conditions in these months in Sohag. Finally, the lowest prevalence of trichostrongyles larvae infection in cattle occurs in winter, with a peak in January.
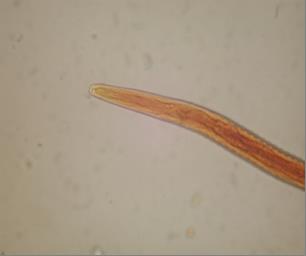
Fig. 1 Anterior end of Haemonchus contortus 3erd stage larve (x400)
TABLE 1
MONTHLY INFECTION RATES OF CATTLE, BUFFALOES AND SHEEP WITH TRICHOSTRONGYLES BY FAECAL CULTURE IN SOHAG, EGYPT
|
Month |
Cattle |
Buffaloes |
Sheep |
||||||
|
Number of examined animals |
Positive cultures |
Number of examined animals |
Positive cultures |
Number of examined animals |
Positive cultures |
||||
|
(No) |
(%) |
(No) |
(%) |
(No) |
(%) |
||||
|
January |
14 |
5 |
35.7 |
15 |
4 |
26.6 |
12 |
7 |
58.3 |
|
February |
15 |
5 |
33.3 |
11 |
3 |
27.2 |
12 |
4 |
33.3 |
|
March |
17 |
11 |
66.7 |
12 |
7 |
58.3 |
15 |
8 |
53.3 |
|
April |
18 |
9 |
50 |
13 |
6 |
46.1 |
16 |
9 |
56.2 |
|
may |
13 |
6 |
46 |
9 |
3 |
33.3 |
5 |
3 |
60 |
|
June |
16 |
11 |
68 |
12 |
3 |
25 |
12 |
8 |
66.6 |
|
July |
11 |
5 |
45.4 |
6 |
2 |
33.3 |
12 |
7 |
58.3 |
|
august |
7 |
3 |
42.8 |
7 |
2 |
28.5 |
8 |
2 |
25 |
|
September |
18 |
8 |
44 |
9 |
3 |
33.3 |
12 |
6 |
50 |
|
October |
17 |
9 |
52.9 |
9 |
4 |
44.4 |
11 |
6 |
54.5 |
|
November |
15 |
8 |
53.3 |
10 |
5 |
50 |
17 |
9 |
52.9 |
|
December |
10 |
3 |
30 |
15 |
2 |
13.3 |
11 |
3 |
27.2 |
|
Total |
171 |
83 |
47.5 |
128 |
44 |
30 |
143 |
72 |
50.3 |
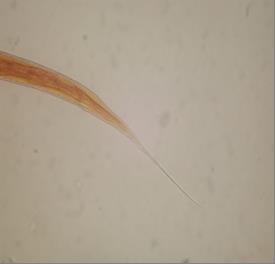
Fig. 2 Posterior end of Haemonchus contortus 3erd stage larve (x400)
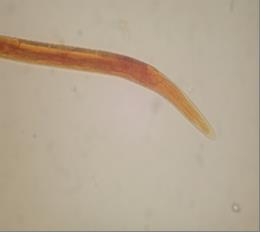
Fig. 3 Anterior end of Trichostrongylus spp. 3erd stage larve (x400)
TABLE 2
SEASONAL INFECTION RATES AMONG CATTLE, BUFFALOES AND SHEEP WITH TRICHOSTRONGYLES LARVAE USING FAECAL CULTURE IN SOHAG PROVINCE
|
Season |
Cattle |
Buffaloes |
Sheep |
||||
|
Number of examined animals |
Positive (%) |
Number of examined animals |
Positive (%) |
Number of examined animals |
Positive (no) |
Positive (%) |
|
|
Winter |
39 |
33.3 |
41 |
21.9 |
35 |
14 |
40 |
|
Spring |
48 |
54.1 |
34 |
47 |
36 |
20 |
55.5 |
|
Summer |
34 |
55.9 |
25 |
28 |
32 |
17 |
53.1
|
|
Autumn |
50 |
50 |
28 |
42.8 |
40 |
21 |
52.5 |
|
Total |
171 |
48.5 |
128 |
34.4 |
143 |
72 |
50.3
|
TABLE 3
INFECTION RATES OF CATTLE, BUFFALOES AND SHEEP WITH DIFFERENT G. I. LARVAE AFTER FAECAL CULTURE
|
Animal spp. |
Cattle (n=83) |
Buffaloes (n=44) |
Sheep (n=72) |
|||
|
The parasite |
(No) |
(%) |
(No) |
(%) |
(No) |
(%) |
|
Coopereia spp. |
41 |
49.3 |
20 |
45 |
12 |
16 |
|
Trichostrogylus spp. |
13 |
15.6
|
11 |
25
|
9 |
12.5 |
|
Ostertagia spp. |
22 |
26.5
|
15 |
34
|
22 |
30.5 |
|
Haemonchus spp. |
15 |
18 |
4 |
9 |
19 |
26 |
|
Strongyloides papillosus |
34 |
40.9 |
19 |
43 |
27 |
37.5
|
|
Oesophagostomum spp. |
5 |
6
|
0 |
0 |
20 |
27.7 |

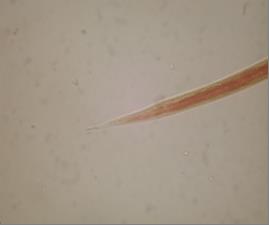
Fig. 4 Posterior end of Trichostrongylus spp. 3erd stage larve (x400)
In buffaloes, the spring season was the most favorable, with infection peaking in March, then autumn, peaking in October, then the summer season, peaking in July, and finally winter, peaking in February. This agrees with the observations of (Abdel-Wahed 1987 and Sobhy 2005).
There was little difference with sheep than with the other two. The most favorable season for the infection is spring, peaking in May, followed by summer, peaking in June, autumn, peaking in October, and then winter, peaking in January.
These results agree with (Fakae 1990; Abdel-Wahed 1987; and Sobhy 2005). They state that the summer season is the most favorable one for trichostrongyle sp. infection. Torina et al (2004) states that preliminary data shows the maximum peak of egg production is during the winter period. This is in contrast with other countries, where winter is a period of hypobiosis.
In conclusion, the current study reports a high infestation rate of all ruminants in Sohag, Egypt, with abomasal worms. Aperiodic parasitology checks of ruminant animals and the application of suitable treatment is strongly recommended.
References
1. Abdel gawad AF 1972: Differential diagnosis of G.I.Strongylosis of sheep in Egypt through the free living larvae.10th Arab vet. Congress, 12-19 Feb., pp.212-227
2. Abdel-Wahed MM. 1987: Morphological studies on G. I. nematodes infesting buffalo in Kalubia and Sharkia Governorates. M.V.Sc. Thesis, Cairo University.
3. Burger HJ and Stoy, M 1968: Parasitologsche diagnostic (teil 11): Elzahlung und larven differenierung.Therapogen praxisdienst, 3: 1-22.
4. Charles MH 1998: Diagnostic veterinary Parasitology. Second Edition. Page-254.
5. Chollet JY, Martrenchar DB and Njoya A 1994: Epidemiology of digestive parasitic diseases of young cattle in northern Cameroon. Rev Elev. Medical Veterinary Pays Tropical. 47:365-74.
6. Corwin R. 1997: Economics of gastrointestinal parasitism of cattle. Veterinary. Parasitology 72, pp. 451–457.
7. Eckert AM 1960: The diagnosis of gastro intestinal Strongylosis in the sheep by the differentiation of the free living third stage larvae).Zentrbl Veterinary, medical journal 94:612-629.
8. Ezzat MA 1960: The geographical distribution and incidence of important parasitic diseases in Egypt and its bearing on the livestock production. Journal Arabian Veterinary Medical Association. 20:127-136
9. Fakae BB 1990: The epidemiology of helminthosis in small ruminants under the traditional husbandry system in eastern Nigeria. Veterinary Research Communication, 14:381-91.
10. Guirgis RA 1984: Egyptian Sheep Resources. Animal Genetic Resources Information (AGRI), Bulletin 13, FAO Publisher, pp: 47-58.
11. Hanelein GF and Abdellatif MA 2003: Trends in small ruminant husbandry and nutrition and specific reference to Egypt. Small Ruminant Res., 15: 185-200
12. Sultan K, Desoukey AY, Elsiefy MA and Elbahy, NM 2010: An Abattoir Study on the Prevalence of Some Gastrointestinal Helminths of Sheep in Gharbia Governorate, Egypt. Global Veterinaria 5 (2): 84-87, 2010 ISSN 1992-6197
13. Khalaf-Allah SSM. 1994: Epidemiological studies on G.I.nematodes in some ruminants.Ph.D. Thesis fac. Vet. Med. Zagazig University.
14. Nagaty HF, Fahmy MA and Hegab SM 1947: New records on some parasites from Egyptian food mammals. 30: 217-218.
15. Perry BD and Randolph TF 1999: Improving the assessment of the economic impact of parasitic diseases and of their control in production animals. Veterinary Parasitology, 84: 145-168.
16. Richard LG and, Zimmerman GL 1992: The epizoology of G.I.Nematodes of Cattle in selected area of Oregon. Veterinary Parasitology 43: 271-291.
17. Sobhy SG 2005: Some studies in helminth parasites of abomasum of cattle and buffaloes in Kafr-Elsheikh province. M.V.Sc. Thesis, Kafr-Elsheikh University.
18. Torina A, Ferrantelli, V, Sparagano OA, Reale S, Vitale F. and Caracappa S. 2004: Climatic conditions and gastrointestinal nematode egg production: observations in breeding sheep and goats. New York Academy of Sciences 1026:203-209.
© 2016 The Author(s). Published by All-Russian Scientific Research Institute of Fundamental and Applied Parasitology of Animals and Plants named after K.I. Skryabin. This is an open access article under the Agreement of 02.07.2014 (Russian Science Citation Index (RSCI) http://elibrary.ru/projects/citation/cit_index.asp) and the Agreement of 12.06.2014 (CA-BI.org/Human Sciences section: http://www.cabi.org/Uploads/CABI/publishing/fulltext-products/cabi-fulltext-material-from-journals-b...)